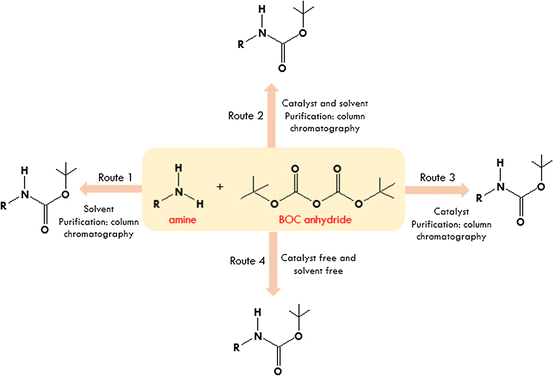
Efficient and expeditious chemoselective BOC protection of amines in catalyst and solvent-free media | SpringerLink

Deprotection of N‐tert‐Butoxycarbonyl (Boc) Protected Functionalized Heteroarenes via Addition–Elimination with 3‐Methoxypropylamine - Gulledge - 2020 - European Journal of Organic Chemistry - Wiley Online Library

Advanced Organic Chemistry: Reaction Mechanism, Strategy, Applications.... - Advanced Organic Chemistry: Reaction Mechanism, Strategy, Applications.

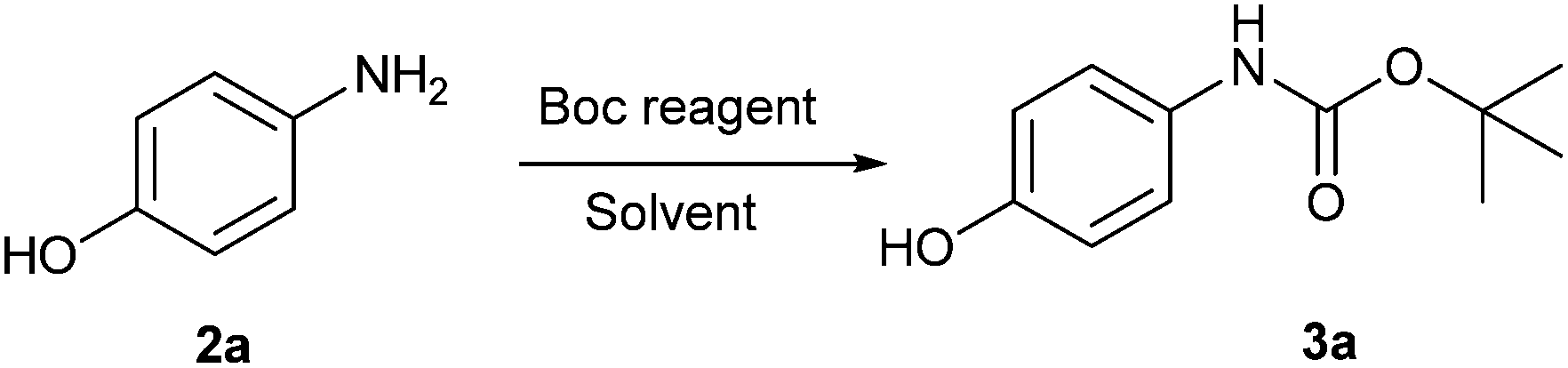
N-tert-Butoxycarbonylation of Structurally Diverse Amines and Sulfamides under Water-Mediated Catalyst-Free Conditions
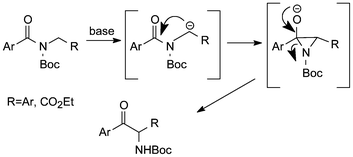
Dual protection of amino functions involving Boc - RSC Advances (RSC Publishing) DOI:10.1039/C3RA42956C

An efficient and highly chemoselective N-Boc protection of amines, amino acids, and peptides under heterogeneous conditions | SpringerLink